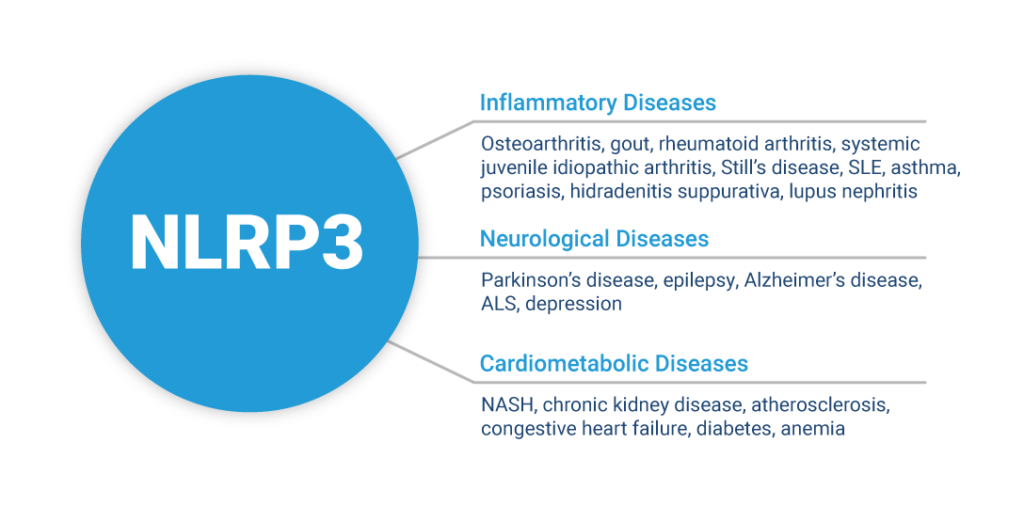
NLRP3 is the best understood member of a family of proteins known as inflammasome receptors. When inactive monomeric NLRP3 senses intracellular damage, it oligomerizes into its active conformation, forming a highly organized inflammasome complex. This inflammasome complex leads to the activation of IL-1β, IL-18, and Gasdermin D (GSDMD) to promote a downstream inflammatory response as well as pyroptosis, a type of cell death triggered by pro-inflammatory signals and associated with inflammation.
NLRP3 is activated by numerous markers of tissue damage, such as abnormal protein aggregates and the accumulation of lipids. All of these are known triggers of disease. Because NLRP3 can be activated by such a broad and diverse set of markers of tissue damage, it is a critical regulator of the innate immune response in systemic inflammation and neuroinflammation.
Aberrant activation of the NLRP3 inflammasome is a key driver in many diseases
NLRP3 was first recognized as playing an essential role in human disease with the discovery that activating mutations of NLRP3 cause a group of genetic auto-inflammatory diseases known as cryopyrin-associated periodic syndromes (CAPS). These diseases are characterized by IL-1β-mediated systemic inflammation and clinical symptoms involving the skin, joints, central nervous system, and eyes. NLRP3 is also implicated in a broad spectrum of disease pathologies, including: